a collection of 1.25×1019 electrons has the charge of|A collection of 1.25Ã1019 electrons has the charge of?answers : Clark Expert-Verified Answer. question. No one rated this answer yet — why not be the first? 😎. usmanbiu. Answer: -2 C. Explanation: charge of an electron = - therefore .
We've compiled a couple of Pokemon Omega Ruby cheats that you can try out and see if they work with the right version of the ROM that you have. . Change the first Y to 0 for Regular Pokemon or 1 for .
a collection of 1.25×1019 electrons has the charge of,A collection of 1.25×1019 electrons has the charge of -3c, -1c, or -2c Your solution’s ready to go! Our expert help has broken down your problem into an easy-to-learn solution you .
Find step-by-step Chemistry solutions and your answer to the following textbook question: A collection of 1.25×10^19 electrons has the charge of -3c, -1c, or -2c.
The correct option is C -2 C. Number of electrons, n= 1.25×1019. Charge on one electron, q =−1.6×10−19C. ∴ Total charge on n electrons =nq = 1.25×1019×(−1.6×10−19) Hence, .
Q = (1.25 * 10⁻¹⁹ ) (-1.602 * 10⁻¹⁹)C/electron. Q = -2.003 C. Therefore, the collection of 1.25 * 10⁻¹⁹ electrons has a total charge of approximately -2.003 .
A user asks how to calculate the charge of a group of 1.25x10¹⁹ electrons from the electron charge and Avogadro's number. An expert answers with the formula and the result, and . Expert-Verified Answer. question. No one rated this answer yet — why not be the first? 😎. usmanbiu. Answer: -2 C. Explanation: charge of an electron = - therefore .a collection of 1.25×1019 electrons has the charge ofA proton is a positively charged particle located in the nucleus of an atom. An electron has \(\mathrm{\frac{1}{1836}}\) times the mass of a proton, but an equal and opposite .A collection of 1.25\times 1019 electrons has the charge ofGroup of answer choices-1 Coulomb-3 Coulom. Your solution’s ready to go! Enhanced with AI, our expert help .
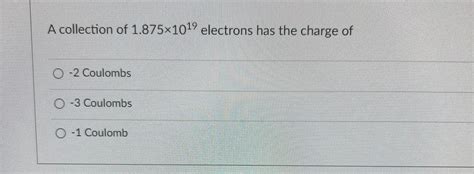
a collection of 1.25×1019 electrons has the charge of A collection of 1.25Ã1019 electrons has the charge of?answers Coulomb(C) is the SI unit of charge. Hence it can be used the express the charge possessed by any body, not necessarily a proton or electron. In this case, a proton possesses a charge of .(1) (1) (1) we can determine the charge of the collection: Q = 1.875 × 1 0 19 ⋅ 1.6 × 1 0 − 19 C = 3 C. Q=1.875 \times 10^{19}\cdot 1.6 \times 10^{-19}\text{ C}=3 \text{ C}. Q = .The charge of one electron is -1.6x1019 C, hence the charge of 1.25x10¹⁹ electrons is (1.6 x 10⁻¹⁹)x (-1.25x10¹⁹) = -2 C. The electron charge, denoted by the symbol e, is a basic physical constant that represents the 1.602176634 1019 coulomb, the naturally occurring unit of electric charge.A collection of 1.875×1019 electrons has the charge of-2 Coulombs-3 Coulombs-1 Coulomb Your solution’s ready to go! Our expert help has broken down your problem into an easy-to-learn solution you can count on.The correct option is C -2 C. Number of electrons, n= 1.25×1019. Charge on one electron, q =−1.6×10−19C. ∴ Total charge on n electrons =nq = 1.25×1019×(−1.6×10−19) Hence, the correct answer is option (c). Suggest Corrections. 2.A collection of 1.25Ã1019 electrons has the charge of?answers A charged particle is held at the center of two concentric conducting spherical shells. Figure 23-33a shows a cross section. The given figure gives the net flux Φ \Phi Φ through a Gaussian sphere centered on the particle, as a function of the radius r r r of the sphere. What are the charge of the central particle ? To calculate the charge of a collection of electrons, we need to know the charge of a single electron and the number of electrons in the collection. The charge of a single electron is approximately -1.602 x 10^(-19) coulombs. Given that the collection has 1.875 x 10^19 electrons, we can multiply the charge of a single electron by the .10/9/2018. 100% (4) View full document. Correct! 1 / 1 pts. Question 2 A collection of 1.875×10 electrons has the charge of 19. -3 Coulombs Correct! -1 Coulomb -2 Coulombs. 0.5 / 0.5 pts Question 3 Rubbing two objects together may cause large number of electrons to be transferred from one object to the other. True Correct!A collection of 1.25×10^19 electrons has the charge of -3c, -1c, or -2c. The magnitude of the charge of the neutron is? During any process, the net electric charge of an isolated system does not change. . Question. A collection of 1.875 × 1 0 19 1.875 \times 10^{19} 1.875 × 1 0 19 electrons has the charge of: a) .
Since we know that the charge of the single electron is e = 1.6 ⋅ 1 0 − 19 C e =1.6 \cdot 10^{-19}\text{ C} e = 1.6 ⋅ 1 0 − 19 C and considering the given number of electrons in the collection, we can calculate the total charge as follows:
Final answer: The charge of a collection of 1.875 × 10¹⁹ electrons is -3 C.Therefore, the correct option is c) Explanation: Each electron carries a charge of -1.6 × 10⁻¹⁹ Coulombs (C).To find the total charge of the collection of 1.875 × 10¹⁹ electrons, we multiply the charge of one electron with the total number of electrons.
Question: How much charge is represented by the given amount of electrons? a. For 6.482 × 1017 electrons, the charge q = mC b. For 2.24 × 1018 electrons, the charge q = mC c. For 4.46 × 1019 electrons, the charge q = C d. For 5.628 × 1020 electrons, the charge q = C. How much charge is represented by the given amount of electrons? Where does the $6.24 \times 10^{18}$ number come from? How was it historically derived? I know that $1$ C $=$ $1$ A s but that just pushes the question down another step, and another and another, at

Physics. Physics questions and answers. 10. Each second, 1.25 x 1019 electrons in a narrow beam pass through a small hole in a wall. The beam is perpendicular to the wall. Using Ampere's law, determine the magnitude of the magnetic field in the wall at a radius of 0.750 m from the center of the beam Permeability of free space 4x 10 'TmA.

A coulomb of charge is the charge associated with 6.25 × 1018 electrons. A coulomb of charge is the charge associated with 1.6 × 1019 electrons. A coulomb of charge is the charge associated with half the charge of 6.25 × 10 -18 electrons. A coulomb of charge is the charge associated with 1.6 × 10 -19 electrons.
So we'll call the charge Q1. So it's going to be the one Coulomb that it had initially plus the charge in Coulombs resulting from 6.88 times ten to the 18 electrons. And each electron has negative 1.6 times ten to the minus 19 Coulombs charge. And this makes a net charge of negative 0.1 Coulombs. Now if the object gained electrons from a .
A collection of 1. 8 7 5 \ times 1 0 1 9 electrons has the charge of. Here’s the best way to solve it. Created by Chegg. Share Share. The charge of a collection of $1.875 \times 10^ {19. View the full answer. One coulomb charge is equivalent to how many electrons. Solution : We know, 1 electron has a charge of 1.6 × 10⁻¹⁹ C charge. We are required to find the number of electrons present in one coulomb charge. 1.6 × 10⁻¹⁹ C charge is equivalent to 1 electron. 1 C charge is equivalent to electron
Question: Question 2A collection of 1.875×1019 electrons has the charge of-3 Coulombs-2 Coulombs-1 Coulomb. Question 2. A collection of 1. 8 7 5 × 1 0 1 9 electrons has the charge of. - 3 Coulombs. - 2 Coulombs.Assuming that the charges of an electron is 1.6 × 10-19 coulombs, the number of electrons passing through a section of wire per sec, when the wire carries a current of one ampere is: Options. 0.625 × 10 19. 1.6 × 10-19. 1.6 × 10 19. 0.627 × 10-17. Advertisements. Solution Show Solution.
a collection of 1.25×1019 electrons has the charge of|A collection of 1.25Ã1019 electrons has the charge of?answers
PH0 · Unit of charge (Coulombs) (video)
PH1 · Solved A collection of 1.25×1019 electrons has the charge
PH2 · Solved A collection of 1.25\times 1019 electrons has the
PH3 · A collection of 1.25×10^19 electrons has the charge of
PH4 · A collection of 1.25×1019 electrons has the charge of?
PH5 · A collection of 1.25×1019 electrons has the charge
PH6 · A collection of 1.25Ã1019 electrons has the charge of?answers
PH7 · A collection of $1.875 \times 10^{19}$ electrons has the cha
PH8 · 17.1: Overview
PH9 · 1.25 × 1019 electrons constitutes the charge of